This Thesis is dedicated to the spectroscopic studies of Er3+ doped NaLaF4, which is a promising material for obtaining up-conversion luminescence. During the process of up-conversion, the photons with lower energy (usually – infrared radiation) are converted to the photons with higher energy (visible and ultraviolet radiation).
In the NaLaF4:Er3+ luminescence spectrum, Er3+ characteristic luminescence bands with a dominant green luminescence band (4S3/2→4I15/2, 540 nm) can be observed. As Er3+ concentration increases (0.1 – 10 mol%), concentration quenching is observed for the green luminescence band, which is associated with interaction processes between Er3+.
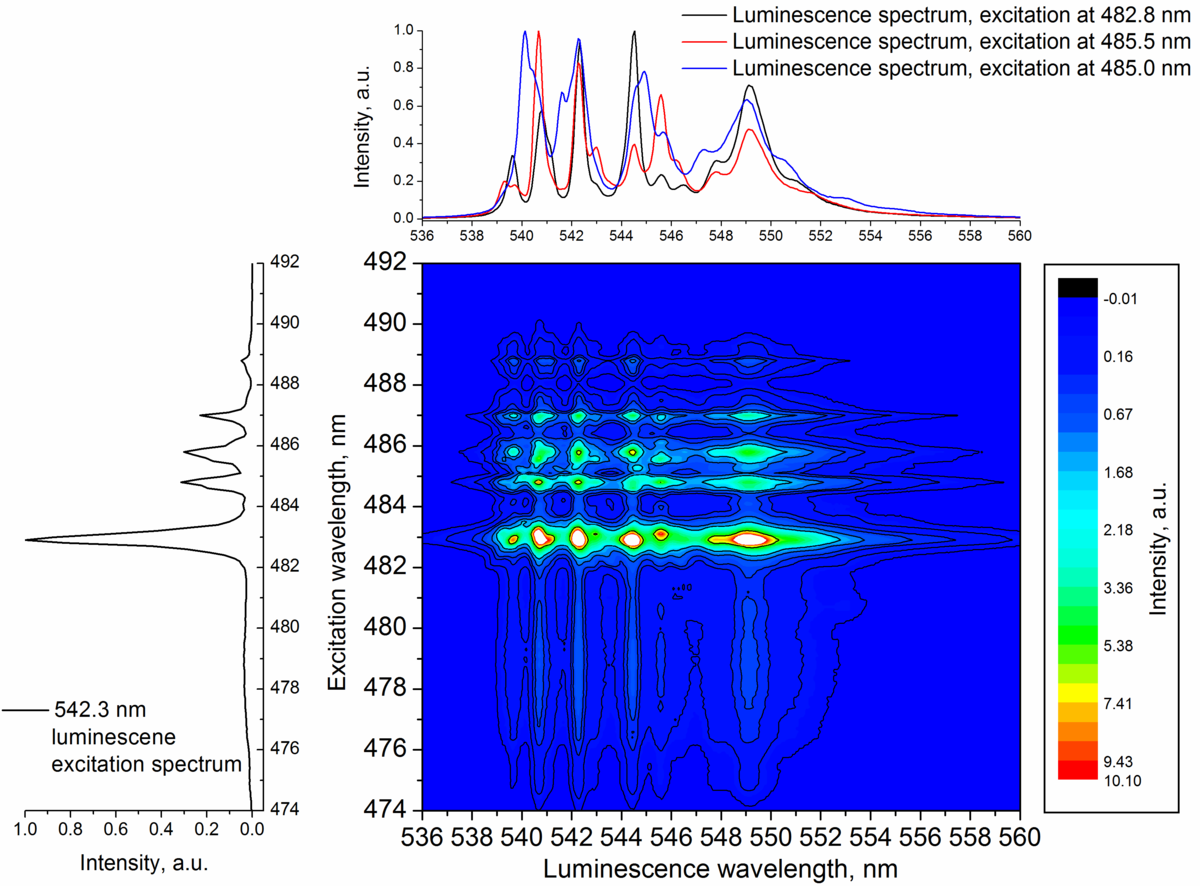
The studies of luminescence spectra and kinetics show that up‑conversion luminescence is excited by two processes: excited state absorption and energy transfer. The contribution of these processes to the excitation of green up-conversion luminescence depends on the excitation wavelength.
Analysis of the measured luminescence spectra and luminescence decay kinetics at different excitation wavelengths (482 – 490 nm) at low temperatures (15 K) confirm that Er3+ can incorporate into several non-equivalent sites in crystal structure of NaLaF4. It is shown that energy transfer occurs between Er3+ located in non-equivalent sites. Up-conversion luminescence spectrum is a superposition of Er3+ luminescence spectra appearing as Er3+ occupies different positions at the crystalline structure of NaLaF4.
Spectroscopic data obtained at different temperatures (15 – 300 K) allow to conclude that apart from 4S3/2 state, an adjacent 2H11/2 state is also involved in Er3+ interaction processes leading to the concentration quenching for the green luminescence.
Key words: luminescence, up-conversion luminescence, NaLaF4, time resolved spectroscopy, site-selective spectroscopy.